
Product introduction
Quiksep prep automatic chromatography system is specially designed for the purification of industrial biological products. It is suitable for the preparation of industrial samples of protein, vaccine, blood products, monoclonal antibody and other biological products.
Quiksep prep adopts the modular and quick disassembly mode, with high degree of automation. It can provide a variety of optional configuration schemes, perfectly meeting the actual production needs of different users.

General description
Stackable pipeline design to achieve the minimum dead volume of pipeline;
To meet the needs of user customization and realize flexible configuration;
The self-developed control software conforms to FDA specifications, with real-time data and log records, traceable and non modifiable;
Flexible method editing function, automatic process production based on time, volume and column volume;
It can realize automatic sample loading and automatic judgment to switch to elution and elution mode after sample loading;
Stable and reliable data acquisition and management function, real-time storage, no loss of power-off data, traceable original records;
Material conforming to USP class VI, protection class IP65;
Complete IQ / OQ file verification.
Product characteristics
1)Meet user customization, flexible configuration
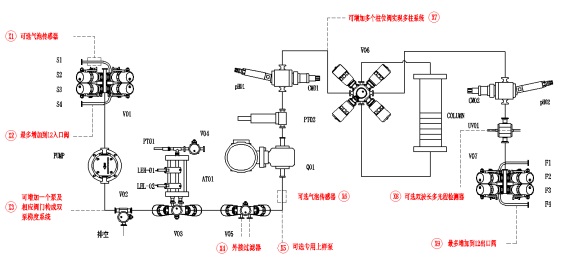
全自动层析系统P&ID图(红色表示可选部件)
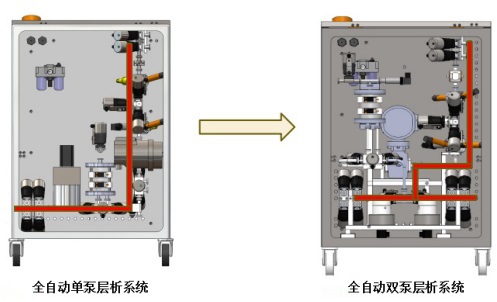
2)Compact structure and ultra small dead volume
The system adopts the design of flow path minimization, sanitary quick loading chuck is connected and fixed, which is convenient and fast, and at the same time greatly reduces the dead volume of the system. The device is very compact, the layout is intuitive and easy to observe. Short response time, good for CIP and sip.

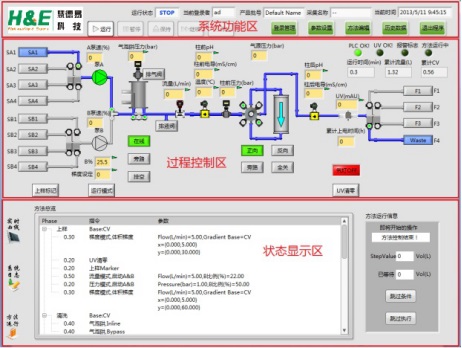
Reasonable and intuitive software operation interface
Quiksep prep automatic chromatography system software conforms to the new GMP specification. It adopts graphical interface and full-automatic operation. It is concise and concise. Any operation and status can be displayed in the main interface status bar in real time, with high degree of automation. Combined with a large number of fault-tolerant design, combined with the actual production, reducing the skill requirements of operators. Reference chart:
4)Customizable entry and exit names
It is beneficial to process design, real-time monitoring of chromatography process and effective avoidance of misoperation.
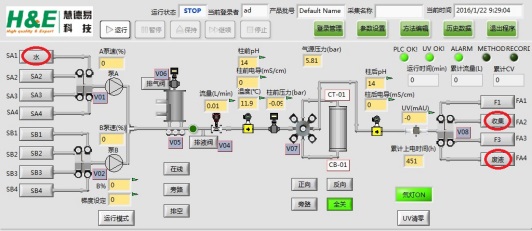
5)The control accuracy is high, and the gradient requirements can be realized accurately
To ensure the accuracy of gradient elution and the beauty of chromatography.
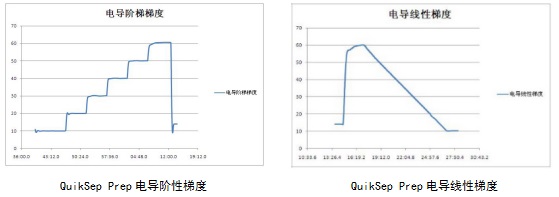
6)Powerful method editing function
It can realize automatic collection according to UV, conductivity value, etc.; it can save temporary editing method automatically; it can run automatic process based on time, volume and column volume; it adopts block method to edit, which makes the method have the function of jump, interrupt and nesting. With strong watch instruction, it can realize many functions, including automatic sample loading, automatic collection and bubble detection Automatic switching and other functions.
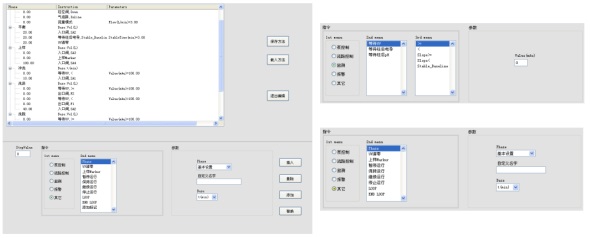
7)Rights management function
The system adopts five levels of user management authority, which can be assigned by users themselves, and users can be changed during operation (convenient for shift handover).

8)Audit tracking and electronic signature function
Perfect audit tracking and electronic signature functions, in line with CGMP regulations.

9)Result analysis and data storage function
The data can be saved in real time, even if the power is cut off, the data will not be lost; the column effect analysis can be carried out for the atlas, and the baseline can be adjusted manually; the system log and method information can be recorded in real time, and the curve comparison can be carried out for the convenience of tracing.

10)Pdf report can be customized
Through the wizard, it is convenient to generate standardized chromatography results report, and to copy and organize data.

四、Main components
1)Diaphragm pump
Low shear force, no inactivation of conveying biological active substances; no pulse, low noise; low failure rate; various specifications are optional: the maximum flow rate can be 1 L / min, 3 L / min, 10 L / min, 20 L / min and other specifications according to actual needs.

2)detector
UV detector adopts imported device, single wavelength (280nm) or variable dual wavelength, to ensure good accuracy and stability.
The pressure sensor adopts international famous brand with high precision, which can provide perfect protection for packing and system pump.
PH / conductivity adopts international famous brand, which can be used for temperature compensation and online calibration.

3)UV circulation pool
The main body of the UV circulation pool has good processability and structural strength, which conforms to FDA and other relevant health certification. The inner cavity of the flow cell follows the design of equal cross-sectional area with the flow passage, which neither increases the flow resistance nor generates additional volume.
External structure diagram:

Internal structure:
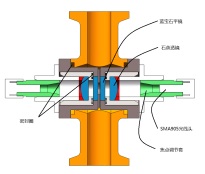
4)On off valve position feedback
The valve switch state of the automatic production type chromatography system needs to be fed back to the control system. The control system depends on the feedback signal to determine whether the flow path meets the expectation, whether all valves work normally, and whether the switching sequence of the combined valve is normal. Therefore, on-off valve position feedback is particularly important in the control system.
The valve position feedback is installed on the pneumatic valve head, fixed with the valve head, with the protection grade of IP65, integrated with the combined pneumatic valve. Built in a trigger switch, status signal light. When the pneumatic valve is opened, the contact will trigger the switch and turn on the status signal lamp at the same time. The valve status can be judged directly from the signal lamp or read from the system control software.
On off valve and feedback diagram:


A pharmaceutical enterprise in Suzhou (2009)

A pharmaceutical enterprise in Xiamen (2013)
![]()

Sichuan vaccine enterprise (2018)